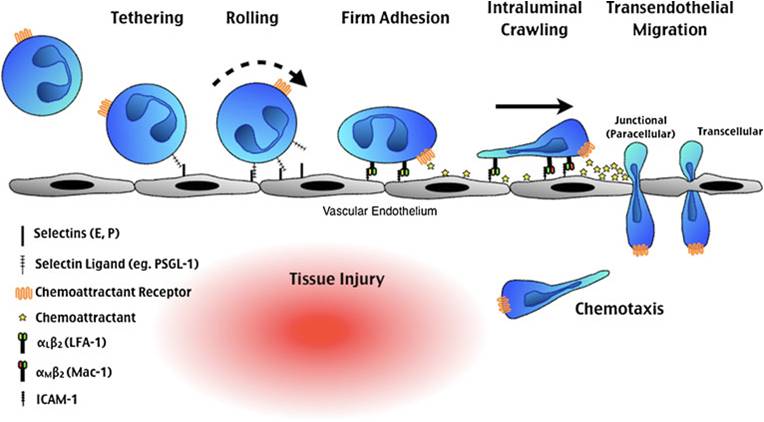
Neuroinflammation is a special form of inflammation that involves the central nervous system (CNS) including the brain and spinal cord as well as, in some cases, the peripheral nervous system (PNS). In the CNS, infection or injury causes migration of white blood cells (WBCs) from blood vessels into the injured tissue across the endothelial or neurovascular blood-brain barrier (BBB). The mechanisms involved in WBC recruitment across the BBB are being intensely studied in many laboratories worldwide. pHLOGISTIX scientists and collaborators are studying the role that high-mobility group box protein 1 (HMGB1) and other damage-associated molecular patterns (DAMPs), important in peripheral tissues, play in WBC recruitment, particularly the polymorphonuclear neutrophils (PMNs), and in BBB transfer. Despite the BBB and other barriers the CNS is subject to a process termed immunesurveillance and vulnerable to immune-mediated pathogenic events.
The figure shows a schematic of how PMNs and other WBCs are obligated to slow down in a process of rolling that causes them to adhere to the endothelial cell (EC) surface and finally to “crawl” to a place between ECs (paracellular) or actually across ECs (transcellular) into the CNS. From McDonald and Kubes (J. Molec. Medicine 89:1079-1088, 2011).
Neuroinflammation is also special since it involves brain-resident cells, the microglia and astrocytes. Both glial cell types participate in inflammatory responses to infection or injury and in neurodegenerative diseases such as Alzheimer’s, Parkinson’s(PD) and Lou Gehrig’s diseases (amyotrophic lateral sclerosis, ALS).
Thus neuroinflammation involves chronic CNS-specific, inflammatory glial responses that may produce neurodegenerative changes, and it may be acutely caused by neural injury wherein microglia migrate to the injured site engulfing dead cells and debris. It may be chronic as with sustained injury wherein the responses of microglial cells contribute to and expand the neurodestructive effects, worsening the disease process.
For example, in traumatic brain injury (TBI), initiation and orchestration of neuroinflammation is complex and multifactorial as it in other tissues, encompassing pro- and anti-inflammatory cytokines, chemokines, adhesion molecules, complement factors, reactive oxygen (ROS) and nitrogen (RNS) species, and other undefined factors.
As in other tissues, it might also be double-edged since injury-induced sterile neuroinflammation can have both beneficial and detrimental effects. The mechanisms underlying this dichotomy are largely unknown.
The pHLOGISTIX approach is that modification of the neuroinflammatory response may be neuroprotective, as in mild-moderate TBI (mTBI) and concussion. Our approach also emphasizes the prognostic value of measuring pro- and anti-inflammatory mediators, as well as molecules reflecting endothelial cell damage in TBI. It should be understood that, with the exception of corticosteroids, no anti-inflammatory agents have been thoroughly tested in TBI, and those that have undergone some testing may have been flawed by their multiple side effects. Consequently, pHLOGISTIX scientists and collaborators are planning clinical trials in mTBI/concussion with anti-inflammatory agents that target multiple, impinging, converging or central and downstream pathways in both adult and pediatric mTBI/concussion.
Of interest, the CNS can also influence inflammation in peripheral tissues. Largely work coming out of groups studying sepsis and involving HMGB1, the prototypic DAMP, Kevin Tracey and colleagues have shown that stimulating the parasympathetic nervous system via the vagus nerve can suppress the magnitude of the proinflammatory response (coining the term inflammatory reflex) since it inhibits HMGB1 release in experimental disease models. pHLOGISTIX scientists and collaborators are studying mechanisms involved in the stress response to injury which is associated with suppressed parasympathetic activity, and activation of the sympathetic nervous system.